Clinical research videos are changing how patients, healthcare providers, and researchers communicate complex medical information. These visual resources break down complicated trial processes, explain treatment options, and share real patient experiences in ways that traditional text simply cannot match.
What Clinical Research Videos Typically Cover:
Videos range from short patient testimonials to comprehensive educational sessions. Major organizations like the National Institutes of Health (NIH), Mayo Clinic, and FDA all use video to educate potential participants. As the NIH INCLUDE Project demonstrates, these videos are particularly powerful for reaching underserved populations and explaining complex concepts like randomization and blinded studies.
I'm Grace Ascione, a registered nurse with an MBA. With over 15 years bridging clinical expertise and healthcare marketing, I've seen how clinical research videos can transform patient understanding and drive meaningful engagement.
When you're considering a clinical trial, you probably have dozens of questions. Clinical research videos answer these in a way that feels personal and real, explaining what it means to be a participant, your role, and what researchers hope to learn. The National Library of Medicine's "What is a Clinical Trial?" video breaks down the basics in plain language, while the American Cancer Society creates videos addressing specific patient concerns like, "How Will I Know If a Trial Is Right For Me?"
This targeted approach is crucial. The NIH INCLUDE Project's webinar for including individuals with Down syndrome in trials saw far more on-demand views than live viewers, showing that people want specific information they can access on their own time. Similarly, regional cancer centers and IBD-focused organizations create videos for their local communities, making participation feel more accessible. At Socorro Marketing, we know that defining your message and brand with medical content marketing requires speaking to specific audiences with relevant, compassionate information.
Nothing compares to hearing from someone who's been in your shoes. The "Voices of Participants Series" from the HHS Office for Human Research Protections features genuine conversations about what trial participation is really like.
Carly, who lives with Crohn's disease, reminds us that today's treatments exist because of past trial participants. You can hear Carly's full story here.
Sara, another Crohn's patient, shares a deeply personal benefit: "It was amazing to feel what I think is normal... The clinical trial got me to the point where I feel healthy enough to be able to carry a baby. It's such a blessing."
Ken, also living with Crohn's, offers advice for those hesitating: "If you are at a point in your disease where you've tried other options... don't let the fear be something that drives you away from health and happiness."
Vishal, who participated in an Ulcerative Colitis trial, captures the dual purpose of participation: benefiting personally while also knowing his "experience... will help others down the line." Watch Vishal's experience here.
These stories create an emotional connection that facts alone cannot, changing the conversation from "Should I?" to "How do I get started?"
While patient stories touch the heart, expert explanations provide the authoritative information needed for an informed decision. Clinical research videos featuring doctors and researchers build credibility and trust.
Dr. Millie D. Long, a gastroenterologist, walks viewers through the evolution of IBD research. Miriam Perez, a research coordinator, answers practical questions that cause anxiety: What happens during a visit? How will the team communicate? What if I get a placebo or my condition worsens? These detailed explanations respect the patient's need to understand the process fully.
The NIH also provides extensive resources, with experts like Dr. Wadih Zein explaining how research advances medical knowledge. These expert perspectives transform clinical trials from an abstract concept into a collaborative effort between patients and professionals working toward a common goal.
When you're considering a clinical trial, the terminology alone can feel overwhelming. Randomization? Double-blind? Placebo-controlled? Clinical research videos are valuable tools because they translate medical jargon into plain language.
These videos walk you through study designs, explaining why researchers use placebos, what randomization means for reliable results, and the safety measures protecting every participant. Resources from the Mayo Clinic and Intermountain Health break down these concepts, clarifying the difference between interventional and observational studies. They emphasize how Institutional Review Boards (IRBs) act as your advocate, ensuring safety and privacy. Understanding this safety net builds confidence. At Socorro Marketing, we've seen how this clarity empowers patients, much like how Content Marketing Can Benefit Your Small Business Healthcare Facility by making complex information accessible.
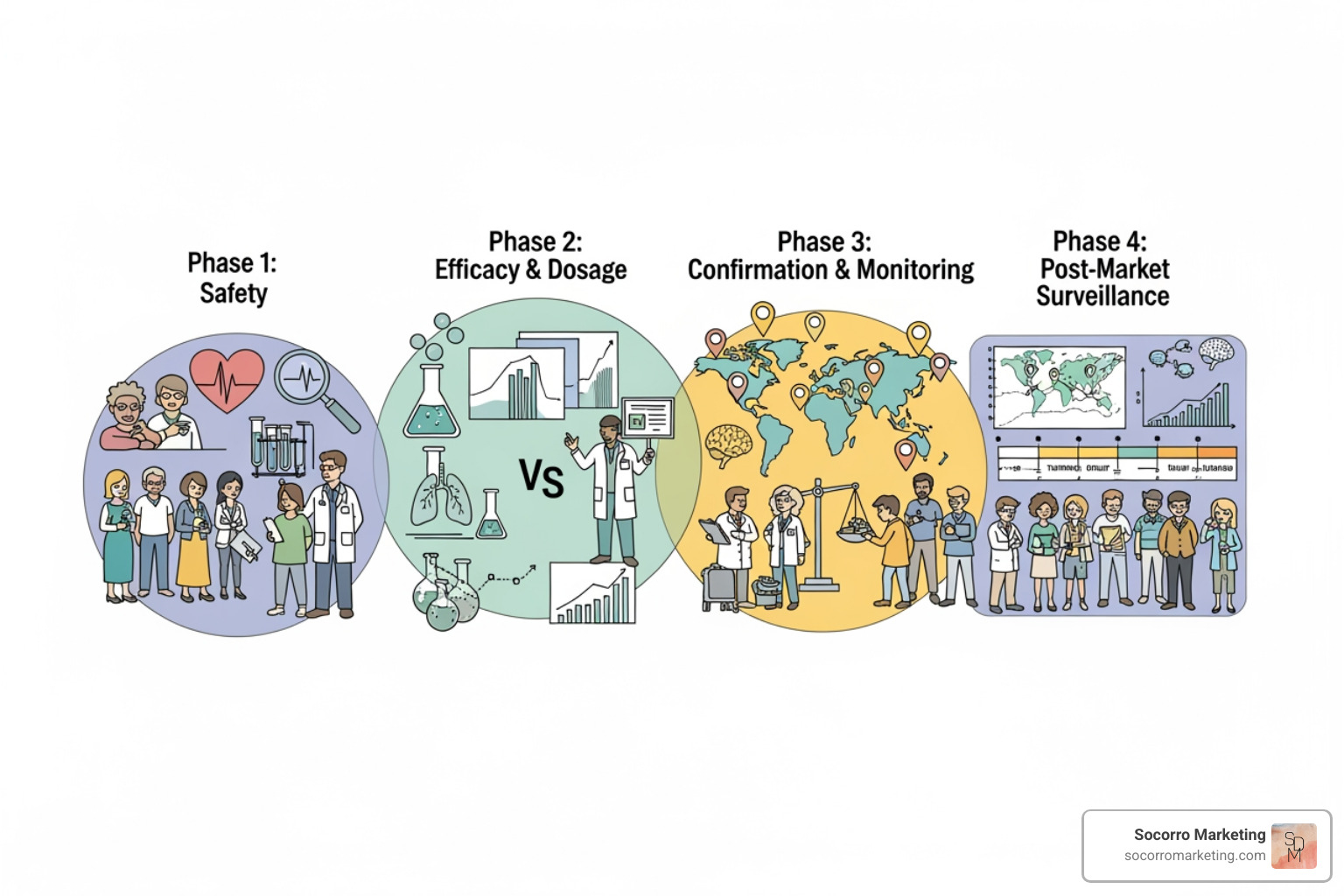
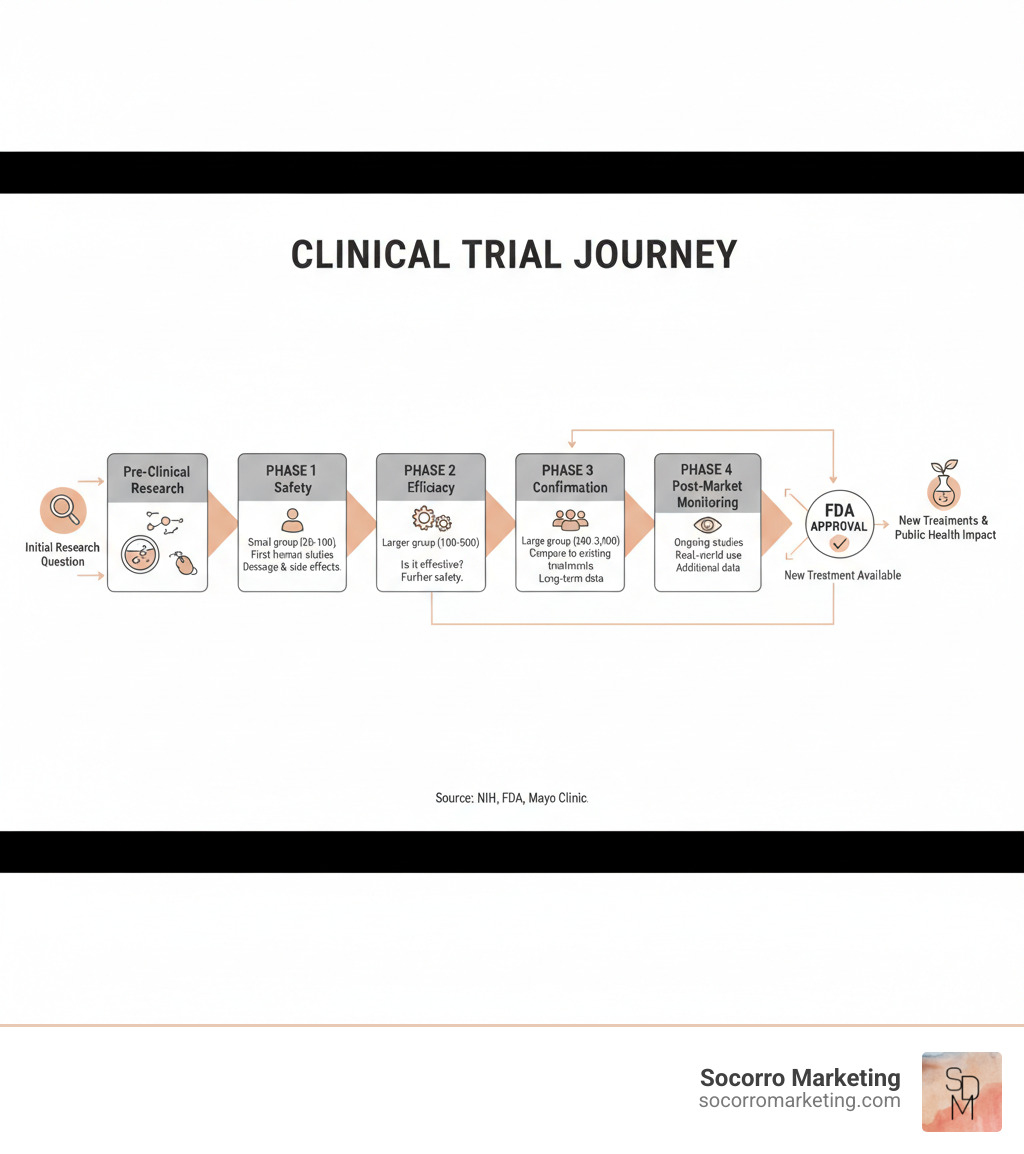
Clinical research videos from the FDA and Mayo Clinic visually map out the four-phase journey every treatment undergoes, a process that ensures safety.
Animations and graphics in videos make this progression easy to grasp, showing that every approved medication has been rigorously tested.
The informed consent process is a crucial conversation before joining a trial. Clinical research videos from the HHS Office for Human Research Protections (OHRP) explain this step clearly.
Informed consent isn't a one-time signature; it's an ongoing dialogue. The research team must explain the study's purpose, procedures, duration, potential risks, and possible benefits. They also must discuss alternative treatments. The consent form is an informational document, not a binding contract. You can ask questions, take it home, and withdraw from the study at any time without penalty.
Behind every trial is an Institutional Review Board (IRB)—an independent committee of scientists, doctors, and community members. Videos show how IRBs act as your advocate, ensuring risks are minimized and your rights and privacy are protected. These safeguards are built on the ethical principles of The Belmont Report. When you see how many people are working to protect participants, it changes the conversation from risk to managed risk for the sake of advancing medicine.
When people hear "clinical trial," fears of being a "guinea pig" or receiving a sugar pill often come to mind. These fears are understandable but largely unfounded. Clinical research videos are remarkably effective at dismantling these misconceptions and building trust.
The beauty of video is its transparency. Seeing a research coordinator explain safety protocols or a patient share their positive experience makes the abstract concept of research tangible and less frightening. For example, in this video discussion on IBD trials, experts address tough questions head-on, replacing myths with facts.

Common Myths vs. Facts in Clinical Trials:
Fear of the unknown is a major barrier. Videos address this by walking viewers through every step, from the first visit to managing potential side effects. When you see that a research coordinator is there to answer questions and coordinate care, you realize you're not alone. Ken, a Crohn's patient, put it perfectly: "don't let the fear be something that drives you away from health and happiness." His words carry weight because he's been there. This message is powerful for patients who feel they've run out of options.
Clinical research videos also highlight the incredible sense of purpose that comes with participation. They show that trials are about people helping people. Vishal, an Ulcerative Colitis patient, felt pride that his experience would "help others down the line." Every available treatment exists because someone decided to participate in a trial. Stories like Sara's, who felt healthy enough to carry a baby after a trial, show the real-world impact. This approach builds trust by connecting viewers to a collaborative effort that benefits both the individual and future generations. At Socorro Marketing, our nurse-managed approach combines this human element with clinical expertise to build lasting trust.
Creating powerful clinical research videos is just the first step. Getting them in front of the right people requires a thoughtful marketing strategy that steers digital distribution and regulatory compliance. Patient recruitment for clinical trials faces significant problems, and traditional doctor-to-doctor referrals are declining. While established agencies like Healthcare Success offer broad marketing services that cover everything from hospital branding to dental SEO, they may lack the niche focus required for complex trial recruitment. Many Clinical Research Organizations (CROs) find they need a more specialized partner, particularly for Phase 2 and 3 trials. This is where Socorro Marketing's nurse-managed approach provides a distinct advantage, combining deep clinical insight with targeted digital strategies like SEO Strategy and Keyword Research to find the patients who could benefit most.
What separates a forgettable video from one that drives action?
Even the best video is useless if unseen. Strategic Digital Marketing ensures your message reaches the right people.
By weaving these tactics together, we ensure that clinical research videos don't just inform—they actively drive patient recruitment, accelerating medical progress.
The journey through clinical research videos reveals a fundamental shift in how medical knowledge is shared. These videos are bridges connecting complex science with everyday understanding, empowering people to make choices that can change their lives and advance medicine for future generations.
For patients, they replace fear with knowledge. Personal stories show that participation is about accessing innovative care and contributing to progress. Expert explanations and transparent discussions about safety protocols build the trust needed to take that first step.
For researchers, these videos are powerful recruitment tools. They streamline patient education and, when paired with strategic digital marketing, reach the right people at the right time. The future of healthcare communication is visual, accessible, and deeply human.
At Socorro Marketing, this work is personal. Based in Golden, CO, we serve healthcare facilities in our home communities across Colorado, Utah, Arizona, and New Mexico. Our nurse-managed approach means we deliver marketing strategies that are not only effective but also medically accurate and ethically sound. We ensure your videos don't just get made; they get seen by the patients who need them most.
Every patient who participates in a trial becomes part of a collective effort to push medicine forward. That's the real power of these videos: they don't just inform, they inspire action.
If you're ready to harness the power of video to advance your clinical research, we're here to help. Learn how to reach more patients with expert behavioral health digital marketing and find what nurse-managed marketing can do for your facility.